A red star is born
 Starphenes are two-dimensional polyaromatic hydrocarbons (PAHs) made up of three acene arms connected via a benzene ring. The foreseen use of starphenes ranges from optoelectronic applications as components in Organic Field-Effect Transistors (OFETs) or Organic Light-Emitting Diodes (OLEDs), to logic gates in single-molecule electronics. Still, starphenes did not reach real applications yet due to their challenging synthesis. In fact, their tendency to interact via p-p stacking make them highly insoluble and only starphenes modified with side groups have been synthesized so far.
Starphenes are two-dimensional polyaromatic hydrocarbons (PAHs) made up of three acene arms connected via a benzene ring. The foreseen use of starphenes ranges from optoelectronic applications as components in Organic Field-Effect Transistors (OFETs) or Organic Light-Emitting Diodes (OLEDs), to logic gates in single-molecule electronics. Still, starphenes did not reach real applications yet due to their challenging synthesis. In fact, their tendency to interact via p-p stacking make them highly insoluble and only starphenes modified with side groups have been synthesized so far.
In this collaborative work between researchers from different European and Japanese institutions, the largest unsubstituted starphene that has been ever synthesized is reported, namely: the planar and fully conjugated [16]-starphene featuring three pentacene arms. The chemical strategy employed makes use of carbonyl protecting groups that are easily removed by thermal annealing, ultimately leading to pristine solid starphenes. The large quantities that can be obtained by this new synthetic route pave the way for implementing large starphenes in optoelectronic devices.
The researchers characterized in detailed ensemble starphene properties with a combination of analytic techniques. The CFM team employed an Atomic Layer Injection (ALI) technique for the first time to deposit such large and fragile molecules dissolved in an acetonitrile solution onto a clean metallic surface and succeeded in studying the electronic properties of single starphene molecules by high-resolution Scanning Tunneling Microscopy (STM). In agreement with theory, starphene exhibits a bandgap (1.64 eV) smaller than pentacene (2.2 eV) and more similar to hexacene (1.76 eV). This implies an enhanced electron delocalization with respect to pentacene. That is, the molecule does not behave as three isolated pentacene arms, but with an electronic coupling between the three arms.
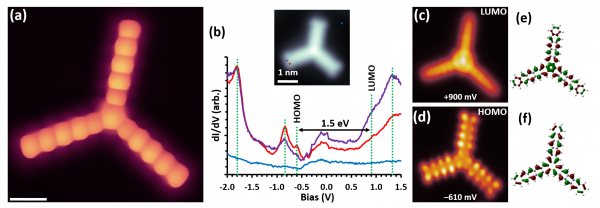
Figure: (a) HR-STM image taken with CO functionalized STM tip that reveals the chemical structure of a single starphene molecule. Scale bar: 5 Å. (b) Differential conductance (dI/dV) spectra measured on a starphene arm showing various resonances marked with dashed lines. The inset marks the positions at which each of the spectra are taken, two on the molecule and one on the substrate as reference. (c) and (d) Constant height dI/dV images of the same starphene recorded with a Cl-functionalized STM tip at the energy of the lowest unoccupied (LUMO) and highest occupied (HOMO) molecular states, respectively. All STM images were recorded at 4.3 K. (e) Calculated LUMO and (f) HOMO of starphene.



