Mechanisms and dynamics of mineral dissolution: A new kinetic Monte Carlo model
Mineral dissolution is a fundamental process in geochemistry and materials science. It is controlled by the complex interplay of atomic level mechanisms like adatoms and terraces removal, pit opening, and spontaneous vacancy creation that can be gradually activated at different energies. Though the development of a comprehensive atomistic model is key to go deeper into the understanding of this phenomenon, existing models had failed to reproduce the abrupt dependence of the dissolution rate with the Gibbs free energy (DG).
In this work researchers from the CFM in collaboration with researchers from Tecnalia R&I and the UPV/EHU have presented the first atomistic model capable of reproducing the experimentally observed sigmoidal dependence of the dissolution rate with the Gibbs free energy. Indeed Martin et al. calibrated their kinetic Monte Carlo (kMC) model to correctly fit the dissolution rates of several representative minerals like albite, smectite, labradorite, alite, or feldspar.
The main novelty of the new kMC model consisted in taking into account the microscopic reversibility of the chemical reactions by adding a new precipitation term to the usual Transition State Theory (TST) dissolution equation. This term turned to be crucial to successfully capture the complex dissolution phenomena. As a such, three different dissolution mechanisms naturally emerged from the simulations depending on dissolving energy and the Gibbs free energy: (i) initial irregularities dissolution at close to equilibrium conditions, (ii) pit opening and step retreat when a critical value of the Gibbs free energy is reached, and (iii) spontaneous vacancy opening at far from equilibrium conditions when the characteristic dissolution energy is low enough. The model also confirmed the generally accepted idea that the onset for the dissolution rate increase is originated by the opening of pits, which constantly supplies terraces for step retreat.
Interestingly, according to the simulations, when the dissolution and precipitation energies are sufficiently low and high respectively, there can exist close-to-equilibrium dissolution modes where spontaneous vacancies creation and pit opening can occur before adatom and terrace removal. These dissolution modes have not been previously reported, and call for experimental attention.
In summary, the work of Martin et al sheds new light on the subtle dissolution mechanisms, and can open the door to the development of a comprehensive theoretical framework for dissolution and other surface-related phenomena like etching.
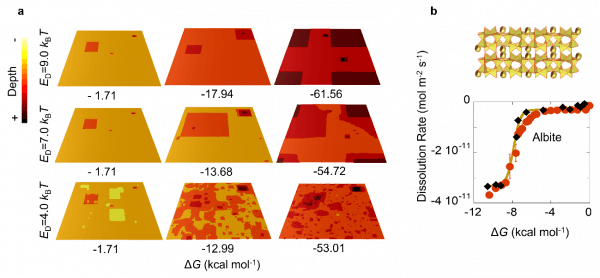
Figure (a) Impact of the dissolution energy (ED) on the surface dissolution patterns obtained for a Kossel crystal close at three dissolution stages: From the left to the right, close to equilibrium, at the dissolution increase onset and far away from equilibrium. (b) Comparison between the experimental rate of an archetypical mineral like albite (black diamonds) with the new kMC predictions (red dots) and empirical fitting schemes (brown line).



