Photocatalytic cofactor regeneration involving triethanolamine revisited: The critical role of glycolaldehyde
The need for efficient conversion of light into useful fuel pushes researchers to find innovative approaches to produce solar fuel. The photo-regeneration of cofactor molecules, using photocatalysts is a cost-efficient strategy of producing added-value chemicals since cofactor can be implemented in many enzymatic reactions.
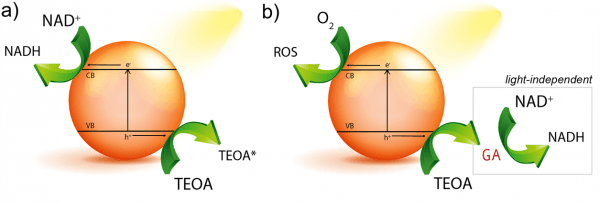
The difficulty in photoregeneration of cofactor molecule remains in the need of using another molecule – sacrificial electron donor- that donates electron(s) to photoexcited photocatalyst to be later passed to the acceptor (NAD+) (Figure 1a). Although water is the target electron donor, it is a chemically stable molecule, requiring the use of alternative solutions. One of such alternatives is triethanolamine (TEOA) that has become a flagship electron donor in research labs since its first implementation in the late seventies. Although its electron-donating character has been demonstrated, little is known on the exact degradation pathway, especially in the process of cofactor regeneration.
Schematic illustrating reduction of NAD+ to NADH
A team of researchers, including Ikerbasque researchers Yury Rakovich (CFM & DIPC) and CSIC researcher Marek Grzelczak (CFM & DIPC), shows that triethanolamine can decompose to glycolaldehyde, an intermediated product that is able of reducing NAD+ to NADH, regardless the presence of light (Figure 1b). These findings show that in the presence of oxygen the degradation of triethanolamine and regeneration of cofactors molecules are not necessarily coupled processes, as has been though for many years. They showed that in the presence of any photocatalyst (conjugated microporous polymer, carbon nitride, platinum nanoparticles and titanium dioxide) and light TEAO decomposes to glycolaldehyde, which induces NADH regeneration in the dark, even after the photocatalyst is removed.
Apart from obtaining a better picture of cofactor regeneration, these basic-research results came with exciting perspectives for future works. It turns out that glycolaldehyde (the simples form of sugar) is of high value in the field of prebiotic chemistry. Glycolaldehyde is an essential intermediate in formose reaction, a process that involves the self-accelerated synthesis of biologically relevant sugars from formaldehyde as a starting material.



