Unfolding the colored centers in nanodiamonds

Nanodiamonds have a crystalline structure of Carbon atoms. Often, this structure contains defects, such as the Nitrogen Vacancy center, which can act as “artificial atoms” which are held in the crystalline structure. These defects can emit light in the same way as normal atoms do.
Diamond is one of the most extraordinary materials on Earth. Its mechanical, electrical and optical properties make it suitable for many technologies. The color of diamond is given by defects on the atomic structure which can be manufactured and behave as “artificial atoms”. In this work, the authors experimentally study the Nitrogen Vacancy center, which has important applications in quantum technologies.
The Nitrogen Vacancy (NV) center in diamond is one of the best know quantum emitters that can be found in diamond. It can be present in both the bulk form of diamond or in nanodiamonds, making it suitable for applications in nanotechnology. It can be used as a biological marker, as diamond is inert and biocompatible. As a quantum emitter, it has been proposed as a potential source of quantum light for quantum communications. In addition, the long coherence of its electronic states makes it ideal as a magnetic quantum sensor. Therefore, the potential of NV centers to become a transformative technology is enormous.
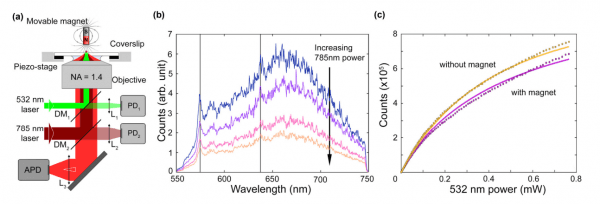
(a) Set-up of the experiment. The nanodiamond is illuminated with different light sources and its emission is collected with an avalanche photo diode (APD) (b) Depletion of the emission of the nanodiamond when more near infra-red light is used (785nm) (c) The emission is affected by magnetic fields, which indicates that the spin dynamics of the NV center plays a central role in the photodynamics.
Nevertheless, one main drawback of the use of NV centers is that the two-level system model for the electronic transitions is only valid in very simple experimental conditions. On the other hand, the dynamics of the electronic states when pumped with different laser frequencies become rather complex. This complexity is enhanced because many of the transition mechanisms are not fully understood. In this experimental work, Roberts et al. perform a series of fluorescence measurements, including different pump wavelengths and external magnetic fields. In this way, they have been able to experimentally fit the different possible physical mechanisms of electronic transitions in the NV.
The main result is that the charge state interconversion, i.e. the NV capturing and releasing an electron, going from a negatively charged state to a neutral one, is responsible for the infrared quenching of the fluorescence of the center. Interestingly, this effect depends on the spin state of the negatively charged state. This has implications for the spin polarization of the NV and has to be taken into account in magnetic sensing applications.



