PhD Thesis Defense | Marina Peña Díaz
 Synergistic study of surface science and electrochemistry: Unraveling the mechanism of oxygen evolution reaction
Synergistic study of surface science and electrochemistry: Unraveling the mechanism of oxygen evolution reaction
October 4, 10:00
CFM Auditorium
Candidate: Marina Peña Díaz
Supervisors: Sara Barja and Celia Rogero
Summary
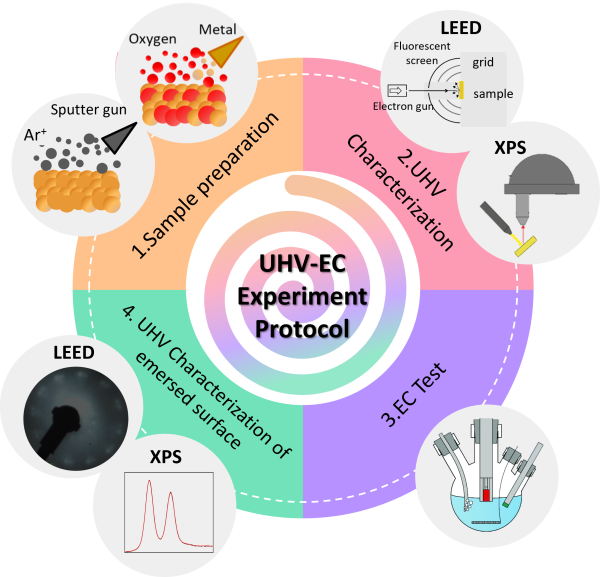
Diagram illustrating the synergistic UHV-EC experiment.
In view of the growing worldwide demand for energy and climate change, the transition towards more sustainable energy alternatives is mandatory. The efficiency and reaction selectivity of electrolyzers and fuel cell devices heavily depend on the chemical and structural composition of catalyst surfaces. Thus, novel catalytic materials with lower overpotential and higher stability and activity for applications in green chemical fuel production and energy conversion are needed. To develop these materials, a comprehensive understanding and monitoring of the electrochemical interface is essential.
This Ph.D. thesis aims to provide comprehensive insights into model electrochemical reactions, such as the electro-oxidation precluding the Oxygen Evolution Reaction (OER), occurring at the electrode catalyst surface/electrolyte interface from a synergistic perspective: electrochemistry and surface science. The research focuses on developing new tools that combine ultra-high vacuum (UHV) conditions with an electrochemical cell to facilitate the examination of the relationship between macroscopic, mesoscopic, and nanoscopic properties—unraveling structure-activity and structure-stability of catalysts evolving the dynamics of the surface and the actual active phase.
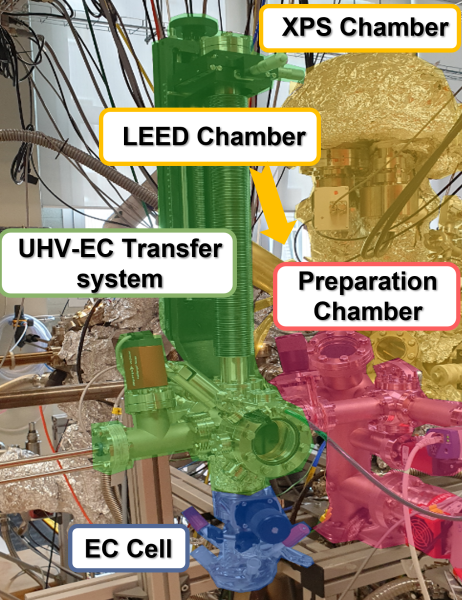
Photograph of the in-house experimental setup designed for quasi-in situ EC-UHV experiemnts




 Synergistic study of surface science and electrochemistry: Unraveling the mechanism of oxygen evolution reaction
Synergistic study of surface science and electrochemistry: Unraveling the mechanism of oxygen evolution reaction