Complexity of confined water vitrification and its glass transition temperature
The precise temperature at which water transforms into glass- a disordered, non-crystalline solid that lacks the long-range order of a crystal yet is rigid like a solid and not fluid like a liquid- remains a mystery because water tends to crystallize at low temperatures.
This temperature, known as the glass transition temperature (Tg), is crucial for various applications, including organ preservation, food freezing, and even insights into climate. This is due to the fact that, similar to conditions in the upper atmosphere, especially in polar stratospheric clouds, water can exist in amorphous (non-crystalline) forms, including glassy water. These amorphous ice structures can influence chemical reactions, radiative transfer, and phase transitions, each contributing to the climate system. Thus, understanding the Tg of water is essential for enhancing our climate process models and making more precise climate change predictions.
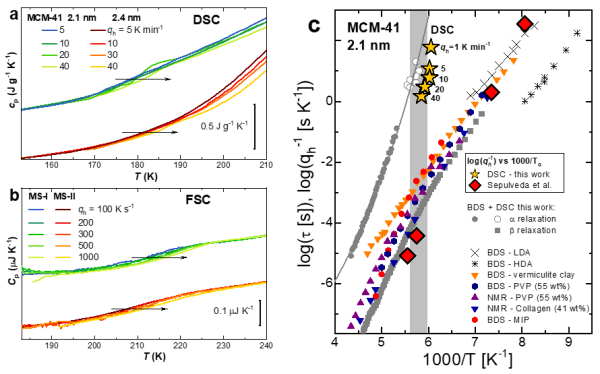
Figure. Dependence of heat capacity with the heating ratio qh for (a) MCM41 (2.1 and 2.4 nm) and MS-I (0.3 nm) and II (0.4 nm). Logarithmic relaxation times of water confined in MCM41 2.1 nm as a function of inverse temperature, compared to the heating rate dependence of the glass transition onset in (a) yellow stars. For comparison, the heating rate dependence of the glass transition onset of bulk water is included as red diamonds. Relaxation times for bulk LDA and HDA water, along with those of water confined in other systems, are added.
Here, we elucidate the glass transition of water by analyzing the calorimetric behavior of nano-confined water across various pore topologies (diameters: 0.3 to 2.5 nm). Our approach involves subjecting the confined water to annealing protocols to identify the temperature and time evolution of nonequilibrium glass kinetics. Furthermore, we enhance this calorimetric approach with the dynamics of confined water, as observed through broadband dielectric spectroscopy and linear calorimetric measurements, including the fast scanning technique. This study demonstrates that confined water undergoes a glass transition in the temperature range of 170 to 200 K, influenced by the confinement size and the interactions with the confinement walls. Moreover, we also illustrate that the thermal event recorded at ~136 K must be understood as an annealing prepeak, commonly referred to as the “shadow glass transition.” Calorimetric measurements also facilitate the detection of a specific heat step above 200 K, which is unaffected by annealing and, therefore, interpreted as a genuine thermodynamic transition. Lastly, by linking our findings to bulk water behavior, we comprehensively understand confined water vitrification.



