Direct observation of desorption of a melt of long polymer chains
A polymer in contact with an inorganic substrate spontaneously forms bonds via Van der Waals forces at the substrate’s surface. Though each of these bonds is intrinsically weak, the presence of numerous pinpoints for each polymer chain results in strong polymer adhesion. This phenomenon, known as polymer adsorption, takes place spontaneously and with reasonably fast kinetics in the polymer melt, underlying the phase transformation from the “standard” unadsorbed to the adsorbed polymer melt, thermodynamically driven by a decrease in the free energy.
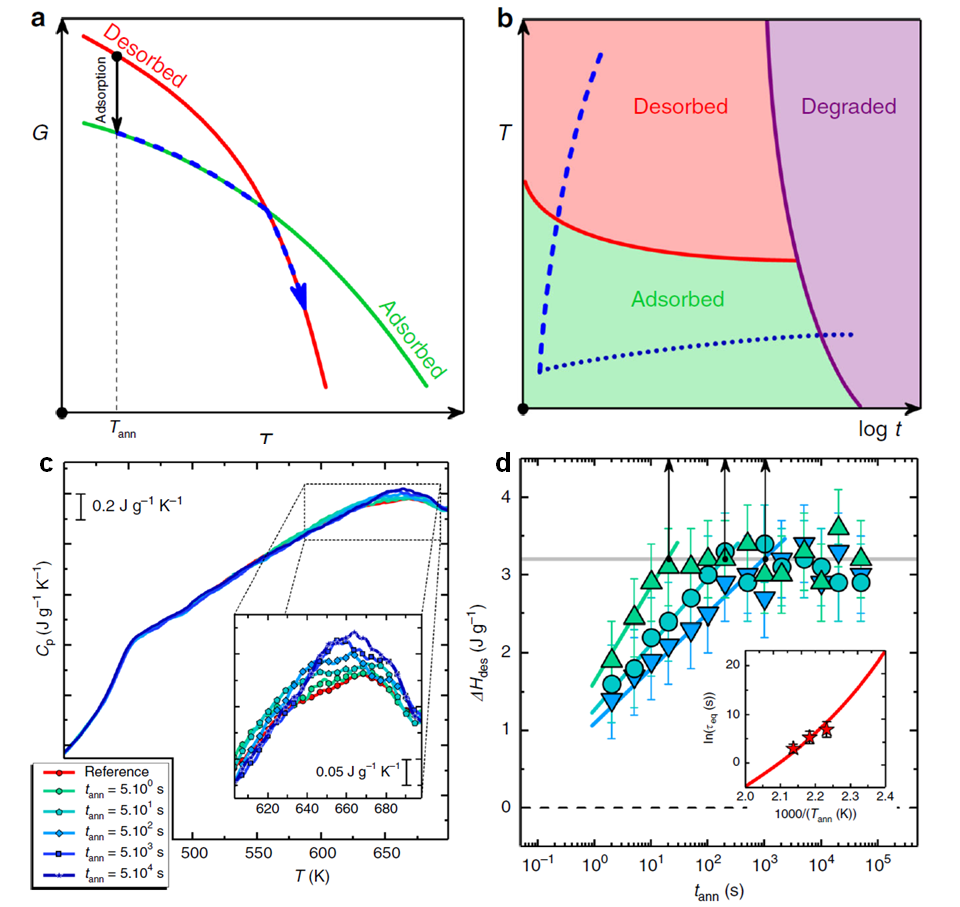
Figure: (a) Free energy vs. temperature for the desorbed (red line) and the adsorbed (green line) phases. (b) Schematic representation of time–temperature–transformation diagram for an adsorbed polymer layer. The blue dashed line and the dark blue dotted line indicate constant heat transformation at, respectively, fast and slow rate. (c) Heating scans at 104 K/s after annealing at 458 K for various times. Inset: enlargement in the temperature range where desorption takes place. (d) Heat of desorption as a function of annealing time, at 448 K (blue), 458 K (cyan) and 468 K (green). Vertical arrows indicate the timescale to reach a plateau in the enthalpy of desorption. Inset: equilibration time as a function of the inverse temperature (stars). The red line depicts the temperature dependence of the relaxation time.
The concomitant decrease of enthalpy and entropy in adsorption is analogous to the well-known phenomenon of crystallization. Within this analogy, increasing the temperature entails the progressive enhancement of the entropic part of the free energy and, thereby, the opposite phenomenon – that is, desorption, analogous to melting, is expected on heating [see panel (a) of the Figure]. Unveiling the existence of this scenario by heating has so far remained elusive due to polymer degradation on heating at low rates [dotted line, panel (b) of the Figure]. To circumvent degradation, this study exploits the capabilities of fast scanning calorimetry allowing heat exchange by a material while the temperature is rapidly varied. With this technique, polymer molecules can be brought to high temperature within a fraction of a second, thus preventing degradation [dashed line, panel (b) of the Figure].
Heating scans at 104 K/s on poly(t-butyl styrene) (PTBS) samples adsorbed for different times above the glass transition temperature are shown in panel (c) of the Figure. The signature of polymer desorption is an endothermic overshoot at about 660 K, underlying the previous adsorption phenomenon. The position of this overshoot is independent of annealing conditions, highlighting the thermodynamic nature of the transition. The magnitude of the overshoot increases with annealing time, signifying increasing amounts of adsorbed polymer. The time evolution of the enthalpy of desorption at different annealing temperatures is shown in panel (d) of the Figure. Decreasing the adsorption temperature renders adsorption slower. Analysis of temperature dependence of the adsorption time [inset of panel (d) of the Figure] indicates that adsorption is triggered by the same process responsible for the glass transition, that is, the primary relaxation of the polymer.
In summary, fast calorimetry permitted to fully characterize the previously elusive phase transition reverting adsorbed polymer chains to standard desorbed polymer melts. This is an important advance on the state of the art of polymer physics. In addition to such advance of the study of phase transitions, this study paves the way to developing new methods that allows tailoring properties of nanomaterials in applications such as smart coatings, flexible electronics and more. The properties of these innovative systems, in fact, depend on how many molecules are adsorbed, and, in this work, Monnier et al. anticipate that by adequately mastering the adsorption/desorption transition it is possible to fabricate better performing and more durable materials.