METAMORPHOSIS OF A COMMODITY PLASTIC LIKE PVC TO EFFICIENT CATALYTIC SINGLE-CHAIN NANOPARTICLES
In addition to recycling, waste valorization by converting polymeric waste materials into more useful products is attracting significant interest to mitigate plastic pollution issues. Polyvinyl chloride (PVC) is the 3rd most widely produced synthetic polymer in the world after the most commonly used polyolefins, polyethylene (PE) and polypropylene (PP). Current global PVC production is estimated at ca. 40 million metric tonnes (Mt) per year. Around 6.5 Mt of PVC are annually manufactured in the European Union (EU) with ca. 2 Mt of post-consumer PVC waste generated.
Mechanical recycling of rigid PVC from the construction and building sector (e.g., windows, pipes) is currently the main relevant recycling process in the EU for post-consumer PVC waste. Recycling or valorization of flexible PVC waste from e.g. packaging, automotive and medical sectors is complicated by the difficulty to handle banned plasticizers (e.g., bis(2-ethylhexyl) phthalate) -still entering recycling streams- after isolation of neat PVC from flexible PVC waste. Currently, significant effort is devoted by many research groups on breaking PVC down to useful products.
This work reports on a complementary concept of polymeric waste valorization (upcycling) by metamorphosis of a commodity plastic of common use in daily life like polyvinyl chloride (PVC) to “valorized” PVC single-chain nanoparticles (vPVC-SCNPs) (Figure, panel a and b). Until now, SCNPs were always synthesized from specialty polymeric precursors and not from waste of commodity plastics. The full valorization process (PVC isolation, PVC azidation, vPVC-SCNPs synthesis) can be run in a green, dipolar aprotic solvent like N-butylpyrrolidinone (NBP) and involving, when required, a simple mixture of ethanol and water (1/1 vol.) as non-solvent. The metamorphosis process when carried out via metal-free click chemistry by means of the Sondheimer diyne as intra-chain cross-linker plus a specific end-capping procedure with benzyl azide leads to well-defined, uniform vPVC-SCNPs that are stable during storage in the solid state for months.
These nanoparticles when loaded with 7.3 mol % of Cu(II) ions -to give vPVC-SCNPs/Cu(II)- become an efficient and recyclable catalyst in the solvent-free homocoupling reaction of polar alkynes, showing enzyme-mimetic behavior in the oxidation of 3,5-di-tert-butylcatechol to 3,5-di-tert-butyl-o-quinone (Figure, panel c) as well as very high turnover frequency (moles of product per mole of catalyst per unit of time, TOF) in the peroxidative oxidation of styrene to benzaldehyde. We envision the use of vPVC-SCNPs/Cu(II) as efficient catalyst in other Cu(II)-catalyzed organic transformations like cyclopropanation, Chan–Lam coupling or the Henry reaction, among other ones. Notably, this new concept is amenable for the valorization of other commodity plastics in which it is feasible to install azide functional groups along their linear polymer chains.
a)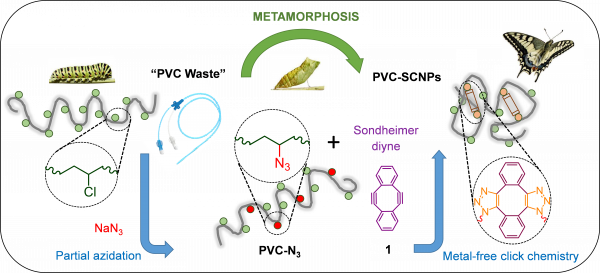
b)
c)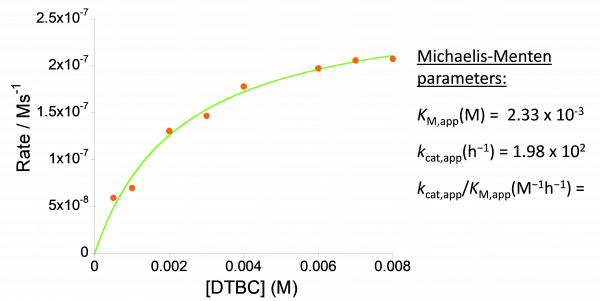
Figure: (a) Illustration of the metamorphosis of “PVC waste” to valorized PVC single-chain nanoparticles (vPVC-SCNPs). (b) The valorization process by starting with an out-of-use PVC hose to give vPVC-SCNPs. (c) Metalloenzyme-mimetic properties of vPVC-SCNPs loaded with 7.3 mol% of Cu(II) ions in the oxidation of 3,5-di-tert-butylcatechol to 3,5-di-tert-butyl-o-quinone.



