A ferromagnetic Eu-Pt surface compound grown below hexagonal boron nitride
Europium is a very interesting element having the ability to occur in a di-, a tri-, or a mixed-valence state. Divalent Eu has a half filled 4f shell and is magnetic while tri-valent Eu is nonmagnetic. We can produce a ferromagnetic Eu-Pt surface alloy by Eu atom intercalation under a 2D hexagonal boron nitride (hBN) monolayer grown on a curved Pt crystal. The hBN coat protects partially the Eu from oxidation, allowing to retain its magnetic properties.
Ferromagnetic two-dimensional (2D) structures are of uttermost importance in spintronics applications. Such 2D magnetic systems can either be 2D van der Waals (vdW) ferromagnets, or ultrathin magnetic overlayers. Both types of systems present quantum and topological phases, but achieving new exotic properties requires design and investigation of novel materials and architectures. In thin magnetic overlayers, transition and/or rare-earth metals are commonly used. The interest in such 2D ferromagnetic systems is prompted by the reduced atomic-scale size and the diversity of magnetic states that arise. Nevertheless, being surface systems they may be subject to oxidation or contamination. One has to find intelligent approaches to protect such 2d ferromagnetic systems from unwanted environmental modifications. Here, we study a hBN-protected ferromagnetic Eu–Pt surface alloy. The Eu–Pt compound is formed after Eu intercalation under the hBN film previously grown on a Pt crystal surface, see Figure 1 for a schematic description of the experiment.
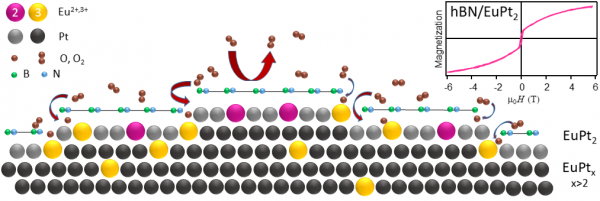
Figure 1: Schematic description of the experimental system. A curved Pt single crystal around the (111) face is used for hBN growth. Eu is intercalated below the hBN forming a ferromagnetic EuPt2 surface compound. After air exposure, the (111) part of the EuPt2 can be protected, at the vicinal parts the protection is strongly reduced.
The substrate material is a cylindrical sector of a single Pt crystal. Across the curved side of the cylinder, the surface features different crystal facets with an increasing density of steps. We expose the substrate hold at 700°C to borazine vapor to form the hBN monalayer. Then we verify the electronic properties by X-ray and angle-resolved photoemission spectroscopy (XPS, ARPES), as well as the structure by low energy electron diffraction and scanning tunneling microscopy. The next step is depositing a small amount of Eu at an elevated temperature. This procedure allows Eu to directly intercalate via wrinkles or grain boundaries of the hBN layer, such as to form an EuPt2 subsurface compound. Our investigations reveal that the majority of Eu stays at the hBN/Pt interface forming the mentioned EuPt2 compound that is ferromagnetic (Curie temperature TC approx. 20 K), with Eu in a divalent state. Part of the Eu further migrates to the Pt bulk forming the non-magnetic, tri-valent Eu-Pt alloy. These results are shown in Figure 2(a) and (b).
Last, we expose this heterolayer system to ambient conditions to check if the very reactive interface Eu atoms are chemically protected by the hBN layer on top. For this, we check again XPS and ARPES at the Pt(335), Pt(111) and Pt(332) facets of the curved Pt substrate, see Figure 2(c). The spectroscopy results in part (d) of Figure 2 indicate that part of the divalent Eu atoms in the EuPt2 interface are protected from air at the (111) and the (335) positions.
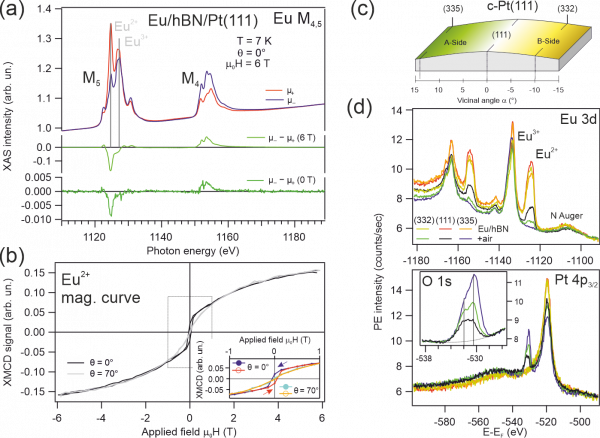
Figure 2: Magnetic and electronic properties of EuPt2 surface compound protected by hBN. (a), (b) X-ray absorption and X-ray magnetic dichroism spectra and magnetization curves at the Eu M4,5 absorption edge confirming the ferromagnetic state of Eu. (c) Schematic description of the curved substrate. (d) X-ray photoemission spectroscopy prior and after air exposure of the hBN/EuPt2 system, revealing partial chemical protection at (111) and (335) surfaces.