An in situ approach to entrap ultra-small iron oxide nanoparticles inside hydrophilic electrospun nanofibers with high arsenic adsorption
Water is a critical liquid on our planet since our biology, chemistry, and other physiological characteristics are based on it. Furthermore, water is a human right and is included as one of the global sustainable development goals (SDGs) for 2030. However, one in three humans worldwide does not have access to safe drinking water. Millions of people in developing and developed countries are affected by drinking water contaminants, particularly emerging contaminants such as heavy metals or pharmaceuticals.
Arsenic is considered one of the most dangerous hazards in drinking water, and at least 230 million people in 108 countries have been drinking water containing arsenic at levels above the WHO provisional guideline value of 10 μg/L. The problem of arsenic contamination in water demands sustainable, scalable, and easy-to-implement solutions. Diverse nano-adsorbents prospered in the last decade, but their use alone requires additional filtering processes to avoid environmental contamination. This work presents a simple, efficient, eco-friendly approach to overcome this drawback while maximizing adsorption capacity.
Researchers from CFM and the University of Buenos Aires (Argentina) developed a novel approach to synthesizing ultra-small nanoparticles (IONPs) within electrospun hydrophilic poly (vinyl alcohol) (PVA) nanofibers, avoiding NPs release into the environment when submerged in water. The in-situ synthesis favour enhanced arsenic adsorption capacity due to the excellent dispersion, tiny size, and surface availability of IONPs, reaching 3.5 mg/g at 10 μg/L. We show that IONPs alter the polymeric matrix properties (such as the glass transition and crystallinity) by preventing the formation of strong hydrogen bond inter/intramolecular interactions of PVA. Insolubility and swelling capacity are essential characteristics of this membrane, which allow solution interchange for arsenic adsorption onto IONPs. Isotherm studies show that the increase from 1 wt% to 3 wt% of IONPs content decreases the active sites for adsorption per mass of IONPs. Still, it does not alter the reusability of the membrane, which reaches at least three adsorption cycles with 80 % efficiency.
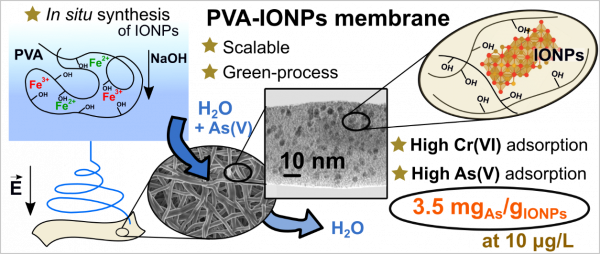
Schematic description of the PVA-IONPs adsorbent adsorbent developed.