ARTIFICIAL PHOTO-SYNTHASES: SINGLE-CHAIN NANOPARTICLES WITH MANIFOLD VISIBLE-LIGHT PHOTOCATALYTIC ACTIVITY FOR CHALLENGING “IN WATER” ORGANIC REACTIONS
In nature, only three types of enzymes carry out purely photocatalytic organic reactions. Indeed, designing abiotic protein nanoreactors capable of custom photoreactions requires extensive genetic engineering effort. In organic photochemistry, scarce solubility of reactants in aqueous media and severe catalyst deactivation is of central concern for the replacement of organic solvents with water. While several strategies have been recently proposed, only a few works have been devoted to the use of ultra-fine soft nano-objects as efficient visible-light photocatalysts of “in water” organic reactions.
Single-chain nanoparticles (SCNPs) -as intramolecularly self-folded synthetic polymer chains with ultra-small size (2-20 nm)- are posed as perfect candidates for advanced, next-generation enzyme-mimetic catalysts preparation. Despite the extensive use of SCNPs as nanoreactors for a plethora of organic reactions, only a few works disclosed the use of SCNPs for photocatalytic applications.
The present work reports the construction of unimolecular soft nano-objects endowed with broad, manifold photocatalytic activity in water and constructed by taking advantage of the protein-mimetic architecture of polymeric SCNPs. These Artificial Photo-Synthases (APS) are used to perform a collection of four organic transformations in aqueous solution at room temperature and under LED illumination (λmax = 450 nm): two reactions unprecedently reported in water, namely, [2+2] photocycloaddition of vinyl arenes, and α-arylation of N-arylamines -which since its discovery by McMillan (2021 Nobel Laureate in Chemistry) was never reported in water-. Additionally, these APS allowed aerobic oxidation of 9-substituted anthracenes and β-sulfonylation of a-methylstyrene (see Figure).
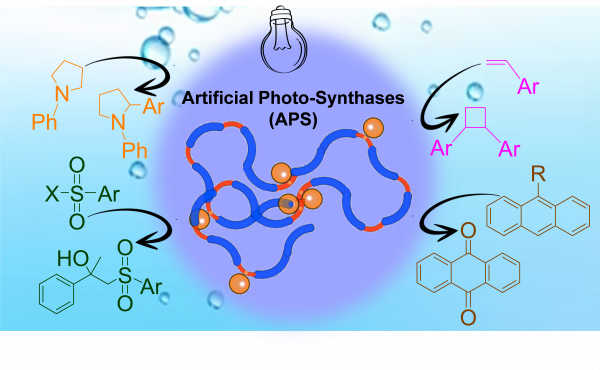
Figure: Illustration of an Artificial Photo-Synthase (APS) endowed with manifold photo-catalytic activity towards “in water” reactions.
Due to the similarities of these APS to enzymes, kinetics data of the [2+2] photocycloaddition of vinyl arenes in water photocatalyzed by APS were analyzed in terms of the classical Michaellis-Menten model. The apparent values of kcat and KM obtained were 2.6 s-1 and 4.6 × 10-2 M, respectively. Comparatively, Chymotrypsin shows kcat = 0.14 s-1 and KM = 1.5 × 10-2 M, Pepsin kcat = 0.50 s-1 and KM = 3.0 × 10-4 M, and tRNA synthetase kcat = 7.6 s-1 and KM = 9.0 × 10-4 M.
In summary, this pioneering work broadens the possibilities for performing challenging “in water” organic transformations via APS-mediated visible-light photocatalysis.



