Can Atomic Buckling Control a Chemical Reaction? The Case of Dehydrogenation of Phthalocyanine Molecules on GdAu2/Au (111)
The efficiency of chemical reactions on surfaces is traditionally related to the atomic structure and catalytic activity of the substrate. Periodic out-of-plane lattice distortions of supported two-dimensional layers is an alternative strategy to promote reactions, strategy that has been scarcely investigated, so far. Here, the authors show that the variable buckling geometry of a GdAu2 Moiré overlayer supported on the Au (111) surface exposes specific single-atom sites that trigger the selective dehydrogenation process of phthalocyanine (H2-PC) molecules. However, a reaction limit to about 1/3 of the monolayer is observed. This self-limited reaction can be explained considering the lattice mismatch between the substrate and the alloy layer, which leads to the previously reported outward displacement of distinct Gd sites.
The detailed knowledge of the surface atomic structures able to promote chemical reactivity is of paramount importance for solid-state nanochemistry. In this work, Vitali et al show that the loss of surface planarity observed in two-dimensional (2D) systems increases significantly the adsorption selectivity and additionally controls the surface chemistry. Specifically, the authors have characterized the reactivity of H2-phthalocyanine (H2-PC) molecules adsorbed on periodic structures characterized by atomic-buckling. The periodic displacement of single atoms orthogonally to the surface plane shown by the GdAu2/Au (111) surface develops spontaneously due to the not commensurable periodicity of the two interfaced structures. Thus, this system is characterized by variable lattice deformations, which reflect the local atomic interaction with the underlying surface. Specific single-atom sites are exposed, allowing them to explore the relationship between the dehydrogenation reaction of the H2-PC molecules and the atomistic structure of the supporting layer. We have demonstrated that this atomic displacement of the Gd atoms in the GdAu2/Au (111) system naturally promotes site selectivity and specificity of the chemical reactions. The energetics of a dehydrogenation reaction of the H2-PC molecules is indeed related to the degree of atomic buckling.
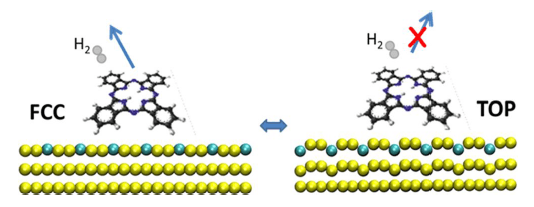
Sketch of the dehydrogenation reaction of the H2-PC macrocycle according to specific corrugation of the GdAu2/Au (111) system. Only when the Gd atoms buckle towards the vacuum region can promote the bonding to the nitrogen atoms of the H2-PC molecule, triggering their dehydrogenation.
By means of scanning tunneling microscopy, X-ray photoemission spectroscopy (XPS), and density functional theory (DFT) calculations, this work gives evidence that the vertical displacement of the Gd atoms is responsible for the dehydrogenation of the macrocycle of only site-selected H2-PC molecules. Thus, at most, one-third of the monolayer (ML) of adsorbed molecules, corresponding to H2-PC molecules occupying well-defined positions of the Moiré superlattice, undergoes a dehydrogenation reaction. The H2-PC dehydrogenation strengthens the Gd−N interaction inducing structural relaxation effects in the alloy geometry. The deformation of this atomistic order may have possible consequences for the stability of the reported in-plane ferromagnetic character of the alloy layer. The present work provides valuable perspective in the selective activation of chemical reactions on surfaces. As atomistic buckling has been commonly reported on several two-dimensional systems, observed as Moiré patterns, the authors believe that these findings might apply generally.



