Confined Water as Model of Supercooled Water
Water is a key compound for the existence of life since it is involved in almost all the chemical, geological and biological processes that occur on our planet. Although water is the most common liquid, it is also the most unusual with many atypical properties. These anomalies become more pronounced in the no man’s land region (150 – 230 K) where bulk water immediately crystallizes into ice. One way to avoid crystallization of water is to restrict the size of water domains by different types of geometrical confinements. In this way, it is possible to experimentally access this temperature region avoiding crystallization. These confinements are also observed in geological systems and in the new nano-technological materials. Moreover, many biological processes take place in very small aqueous environments where water can be considered to be confined in small cavities. Since the presence of confined water is essential for life, the importance of understanding its dynamic and structural properties is extremely relevant.
For this reason, a group of scientists, with different and controversial opinions on the subject of the dynamical behavior of confined water at low temperatures, gathered to give a new vision on this subject. Consequently, this review is not a typical compilation of data previously measured; instead the group of experts provided an integrative approach to this problem. To achieve this challenge, three experimental techniques have been used: dielectric spectroscopy, nuclear magnetic resonance, and quasi-elastic neutron scattering. We have proposed three different scenarios for the dynamics of confined water (see Figure). However, from these studies, it is also clear that the interpretations of the experimental data are far from evident. Therefore, three main interpretations are presented to explain the experimental data, with a discussion of their advantages and disadvantages. Unfortunately, none of the proposed scenarios is able to predict all the observations for supercooled and glassy bulk water, indicating that either the structural and dynamical alterations of confined water are too severe to make predictions for bulk water or the differences in how the studied water has been prepared (applied cooling rate, resulting density of the water, etc.) are too large for direct and quantitative comparisons.
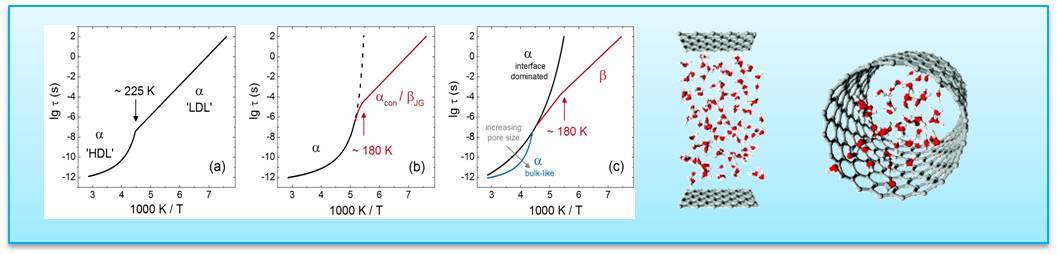 Figure: Schematic diagram at ambient pressure of different scenarios for the dynamical behavior of supercooled confined water. αconf indicate confined α-relaxation, and βJG means Johari−Goldstein β-relaxation. On the right, some confinements used are shown.
Figure: Schematic diagram at ambient pressure of different scenarios for the dynamical behavior of supercooled confined water. αconf indicate confined α-relaxation, and βJG means Johari−Goldstein β-relaxation. On the right, some confinements used are shown.



