DIRECT IMAGING OF COVALENT BOND STRUCTURE IN SINGLE-MOLECULE CHEMICAL REACTIONS
The advances and the refinement of scanning probe microscopy (SPM) have significantly extended the boundaries of molecular imaging. However, even though imaging and manipulation of single atoms in inorganic materials has been routinely performed for many years, comparably high resolution on organic molecules has long remained elusive.
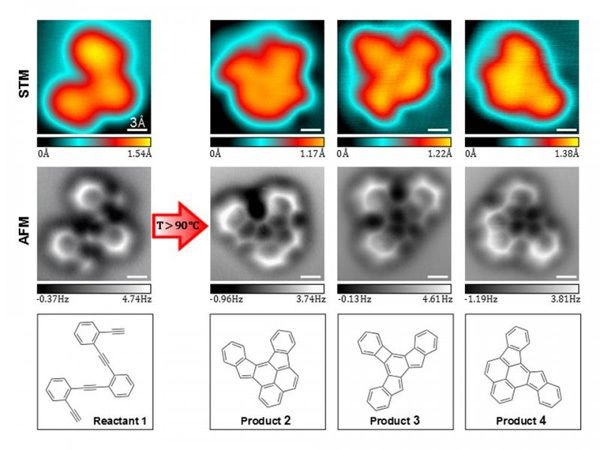
Only recently a new benchmark for SPM has been set demonstrating its capability to directly image the chemical structures of organic molecules by tuning-fork-based non-contact atomic force microscopy (nc-AFM). Key for the enhanced resolution is an appropriate modification of the scanning probe. Functionalization of the tip apex e.g. by individual CO molecules has allowed superior resolution, rendering images reminiscent of wireframe chemical structures in which even differences in bond-order can be identified. The potential of this imaging technique has been demonstrated by the accurate determination of the chemical structure of a natural product that had been previously misassigned based on conventional analytical techniques, or by visualizing the two conformations of a bistable organic molecular adsorbate. In this present work we have taken a substantial step further, resolving for the first time the structural changes and bond rearrangements associated with complex surface-supported chemical cyclization cascades, an accomplishment currently beyond any other experimental technique, including the invasive transmission electron microscopy.
We herein studied a model system based on the thermally induced enediyne cyclization of 1,2-bis((2-ethynylphenyl)ethynyl)benzene (1). This represents an excellent testbed to explore complex chemical transformations within single molecules, and the underlying mechanism of the reaction has important applications for material science. Closely related structures have indeed been explored as a practical route towards the formation of organic functional materials based on high molecular weight poly-acenes. However, a variety of competing chemical processes has been shown to play along the traditional Bergman enediyne cyclization. On the one hand this hampers an accurate prediction of the structure of the cyclized products, and on the other hand the complexity of the resulting product distribution and the inherently poor solubility of the fully-cyclized polyaromatic hydrocarbons often exceed the capability of traditional analytical tools. We tackle this problem running the reaction under highly controlled environment on atomically clean Ag(100) surfaces under ultra-high vacuum (UHV) and by single molecule characterization. This approach has traditionally remained in the realm of scanning tunneling microscopy (STM). However, STM images represent the spatial distribution of molecular orbitals close to EF, additionally broadened and modulated by their hybridization with the electronic states of the substrate (Fig. 1). This leads to a non-trivial contrast in the images that makes the determination of unknown structures a challenging task. Instead, nc-AFM allows direct visualization of the structures of reactant and products (Fig. 1), shedding light onto the transformation reactions. Complementary ab-initio density functional theory calculations further corroborate our experimental results and increase the understanding of the particular reaction pathways.
Hence, in addition to the impact of a direct visualization of chemical reactions, by imaging the complex bond-rearrangements of 1,and complemented withDFT calculations, we provide a detailed mechanistic picture of the cyclization processes previously inaccessible by other experimental tools. In turn, this new insight will guide the design of alternative precursors for the rational synthesis of functional surface-supported molecular architectures.
1Department of Physics, University of California, Berkeley, USA. 2Centro de Física de Materiales CSIC/UPV-EHU, San Sebastián, Spain, 3Department of Chemistry, University of California, Berkeley, USA, 4Materials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, USA, 5Donostia International Physics Center, San Sebastián, Spain, 6Nano-Bio Spectroscopy Group and ETSF Scientific Development Center, Dpto. de Física deMateriales, Universidad del País Vasco UPV/EHU, San Sebastián, Spain.



