EFFICIENT N2 FORMATION ON AG(111) BY ELEY−RIDEAL RECOMBINATION OF HYPERTHERMAL ATOMS
Most reactions occurring at surfaces involve at the latest stage the recombination of two or more species to conform the final molecular compond that desorbs.
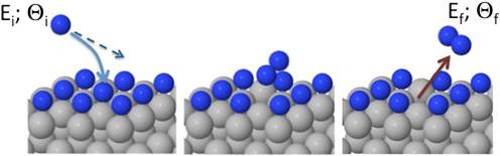
Based on the existing investigations, the common view is that the recombination process proceeds through either chemisorbed species (Langmuir-Hinshelwood recombination), or the hot-atom process in which the incoming gas-phase species experiences few collisions with the surface prior recombining with the adsorbate. The existence of direct pick-up events known as Eley-Rideal recombinations has been usually considered a marginal and rather inefficient phenomenon only observed for incoming light atoms (H,D). In the work published in J. Phys. Chem. Lett. we show that the standard assumption of the Eley-Rideal process as just an exotic and negligible mechanism in the dynamics of gas-metal interfaces can be plain wrong in some systems. By means of molecular dynamics simulations and ab-initio potential energy surfaces, we prove that hyperthermal N atoms can recombine with N atoms adsorbed on Ag(111) in a direct pick up event with an uncommonly high efficiency of more than 35%, even at high incident energies of few eV.



