Evidence of Coupling between the Motions of Water and Peptides
Studies of protein dynamics at low temperatures are generally performed on hydrated powders and not in biologically realistic solutions of water because of water crystallization. Now Izaskun Combarro-Palacios and Silvina Cerveny from CFM (San Sebastian) in collaboration with Jan Swenson from Chalmers University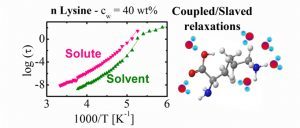 of Technology (Sweden) avoided the problem of crystallization by reducing the size of the biomolecules and studying oligomers of the amino acid l-lysine, fully dissolved in water. The dynamic of these peptides show that the glass transition-related dynamics of the oligomers is determined by the water dynamics, in a way similar to that previously observed for solvated proteins. This implies that the crucial role of water for protein dynamics can be extended to other types of macromolecular systems, where water is also able to determine their conformational fluctuations. Using the energy landscape picture of macromolecules, the thermodynamic criterion for such solvent-slaved macromolecular motions may be that the macromolecules need the entropy contribution from the solvent to overcome the enthalpy barriers between different conformational substates.
of Technology (Sweden) avoided the problem of crystallization by reducing the size of the biomolecules and studying oligomers of the amino acid l-lysine, fully dissolved in water. The dynamic of these peptides show that the glass transition-related dynamics of the oligomers is determined by the water dynamics, in a way similar to that previously observed for solvated proteins. This implies that the crucial role of water for protein dynamics can be extended to other types of macromolecular systems, where water is also able to determine their conformational fluctuations. Using the energy landscape picture of macromolecules, the thermodynamic criterion for such solvent-slaved macromolecular motions may be that the macromolecules need the entropy contribution from the solvent to overcome the enthalpy barriers between different conformational substates.



