Gold as a 6p-Element in Dense Lithium Aurides
The negative oxidation state of gold (Au) has drawn a great attention due to its unusual valence state that induces exotic properties in its compounds, including ferroelectricity and electronic polarization. Although monatomic anionic gold (Au–) has been reported, a higher negative oxidation state of Au has not been observed yet. Here the group at CFM proposed that high pressure becomes a controllable method for preparing high negative oxidation state of Au through its reaction with lithium. First-principles calculations in combination with swarm structural searches disclosed chemical reactions between Au and Li at high pressure, where stable Li-rich aurides with unexpected stoichiometries (e.g., Li4Au and Li5Au) emerge. These compounds exhibit intriguing structural features like Au-centered polyhedrons and a graphene-like Li sublattice, where each Au gains more than one electron donated by Li and acts as a 6p-element. The high negative oxidation state of Au has also been achieved through its reactions with other alkali metals (e.g., Cs) under pressure. This work provides a useful strategy for achieving diverse Au anions.
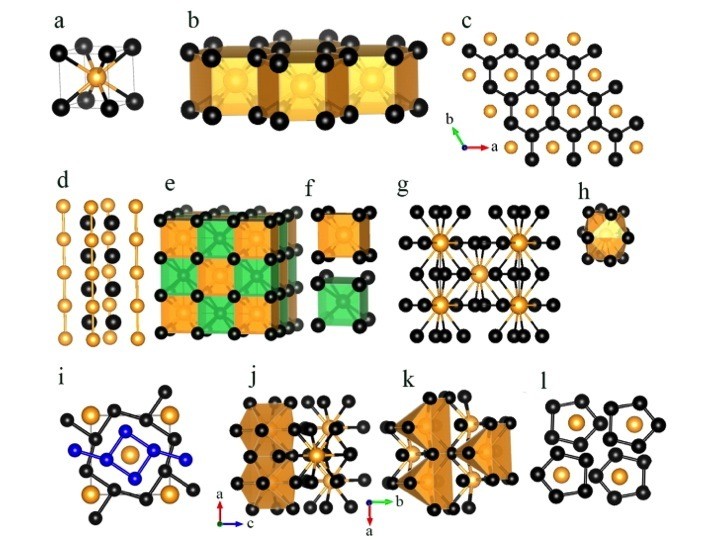
Stable structures of LinAu (n = 1–5) at 50 GPa. The lattice parameters of all of the structures are listed in the Supporting Information (Table S1). (a) LiAu in the Pm-3m structure. (b) Li2Au in the P6/mmm structure. (c) A Li graphene-like layered structure in the P6/mmm structure of Li2Au. (d) Linear Au chains in the P6/mmm structure of Li2Au. (e) Li3Au in the Fm-3m structure. (f) View of hexahedrons in Li3Au. (g) Li4Au in the I4/m structure. (h) View of a dodecahedron in Li4Au. (i) Square Li4 and octagonal Li8 rings in the I4/m structure of Li4Au. (j and k) Li5Au in the Cmcm structure. (l) Planar five-membered Li5 pentagonal rings in the Cmcm structure of Li5Au. In all the structures, small black or blue and large golden spheres represent Li and Au atoms, respectively.



