How adsorbed oxygen atoms inhibit hydrogen dissociation on tungsten surfaces
Hydrogen molecules dissociate on clean W (110) surfaces. This reaction is progressively inhibited as the tungsten surface is precovered with oxygen. Ab-initio molecular dynamics calculations are used here to show that the adsorbed O atoms act as repulsive centers that modulate the dynamics of the impinging H2 molecules by closing dissociation pathways.
Interest in the interaction between hydrogen and metal surfaces has been historically associated with heterogeneous catalysis, the basic route to any large-scale chemical industry production. Hydrogen adsorption on W is one of the simplest chemical reactions that one may envision on a surface. It has been studied for more than 100 years, since Irving Langmuir placed a filament of tungsten in a vessel containing hydrogen gas.
In spite of the extensive coverage of the problem, it is only recently that a basic atomic-level understanding of the mechanisms ruling different reactive processes of hydrogen on W surfaces has been reached. Hydrogen molecules dissociate on clean W (110) surfaces. Experiments show that this reaction is progressively inhibited as the tungsten surface is pre-covered with oxygen.
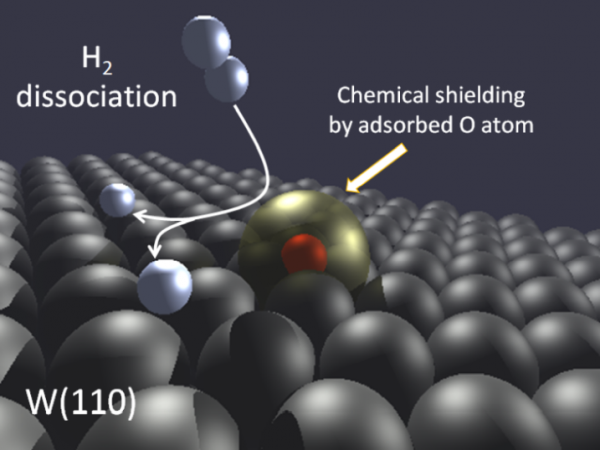
Figure 1: Illustration of the hydrogen dissociation process on W (110) when oxygen atoms are also adsorbed at the surface.
Density functional theory and ab-initio molecular dynamics are used here to rationalize, at the atomic scale, the inhibiting influence of the adsorbed O atoms on the H2 dissociation process. In agreement with existing experimental information, the calculations show that H2 dissociation is absent for an O coverage of half a monolayer. Therefore, the influence of O adsorbates on the dissociation dynamics on W (110) goes much beyond the blocking of possible H adsorption sites. Adsorbed O atoms create a sort of chemical shield at the surface that prevents further approach and dissociation of the H2 molecules. Each O atom creates in its surroundings an exclusion zone for H2 and prevents H2 dissociation on the W atoms located in its close 3-fold vicinity.
The molecular dynamics calculations show that there are actually two types of W atoms on the oxidized surface: those with an oxygen atom in their vicinity and those with no oxygen atom nearby. The former prevent H2 dissociation while the latter provide a path to dissociation in a way similar to the clean surface. This picture may allow to extrapolate the results to other oxygen coverages, provided that phase separation exists.
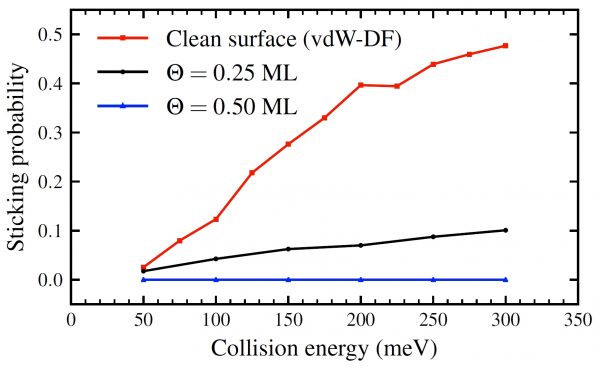
Figure 2: Dissociative sticking probabilities of H2 on W (110) as a function of the collision energy. For all collision energies, the sticking probabilities drop as oxygen coverage of the surface increases.
All in all, ab-initio molecular dynamics calculations are a remarkable tool to understand the intricate dynamics of molecules dissociating over complex systems, such as the oxidized W (110) surface is.



