How viral peptides reshape cell membranes: a soft-matter physics view
Understanding how viruses cross cell membranes is a central challenge at the intersection of soft matter physics, biophysics, and nanoscience. In a recent Nature Communications article, researchers at the Materials Physics Center (CFM–MPC, CSIC–EHU) uncover how short fusion peptides from the SARS-CoV-2 Spike protein self-assemble and cooperatively remodel biomimetic plasma membranes, revealing new physical principles underlying viral entry.
Using well-controlled model membranes that mimic the outer leaflet of the human plasma membrane, the team combined neutron reflectometry, grazing-incidence X-ray diffraction, atomic force microscopy, and infrared spectroscopy to probe peptide–lipid interactions with sub-nanometre resolution. They show that different fusion peptides adopt distinct supramolecular organizations at the membrane interface, forming rigid fibres or flexible spiral assemblies depending on lipid packing and peptide sequence. These assemblies selectively disrupt cholesterol-rich domains, increase membrane fluidity, and store mechanical energy that can be released under compression.
The results support a cooperative, mechano-chemical mechanism for membrane fusion, in which peptide self-assembly converts binding energy into membrane stress and curvature, facilitating pore formation. Beyond providing new insight into SARS-CoV-2 infection, this work highlights how simple peptide motifs can be engineered to sense, store, and release mechanical energy at soft interfaces, opening opportunities for antiviral strategies and for the design of responsive peptide-based nanomaterials.
This study exemplifies the CFM’s strength in applying materials physics concepts and advanced scattering and surface-science techniques to biologically relevant problems, bridging fundamental soft-matter physics with molecular biology and nanotechnology.
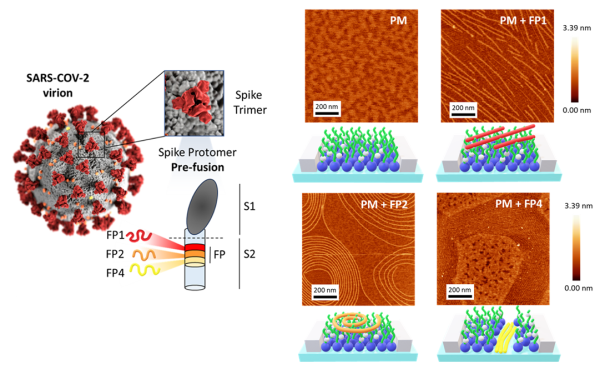
Left: Schematic of the SARS-CoV-2 virion and the Spike protein in its prefusion conformation, highlighting the location of fusion peptides FP1 (red), FP2 (orange), and FP4 (yellow) within the S2 subunit. Right: AFM topographical micrographs and corresponding topographical profiles of Plasma Membrane (PM), PM-FP1, PM-FP2, and PM-FP4. Schematic representations of the corresponding AFM images are also presented for clarity.



