Microscopic Evidence for the Topological Transition in Model Vitrimers
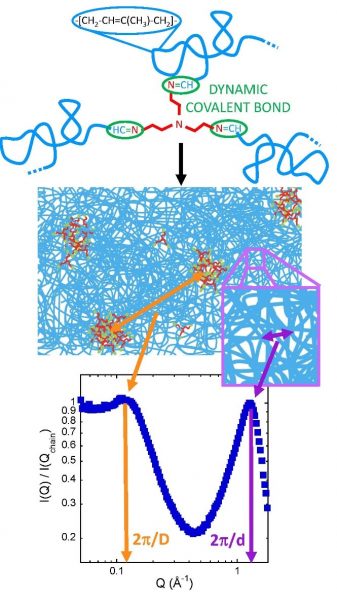
Figure 1: Upper panel: Schematic representation of the vitrimer. Central panel: Drawing of the nano-segregated structure suggested by SAXS. Lower panel: SAXS results corresponding to the lowest molecular weight investigated.
Introduction: Vitrimers consist of permanent networks containing dynamic bonds that allow the topology of the network to change, keeping always constant number of cross-links. In addition to the glass transition, vitrimers undergo a topological transition from viscoelastic liquid to viscoelastic solid behaviour when the network rearrangements facilitated by dynamic bond exchange reactions freeze. The microscopic observation of the topological transition is elusive.
Model polyisoprene vitrimers were investigated. In these dynamic networks nanophase separation of polymer and reactive groups leads to the emergence of a relevant length scale characteristic for the network structure, as observed by SAXS. Clusters of linkers separated by an average distance D give rise to a clear peak in the structure factor, while the correlations between adjacent chains emerge as a peak at much higher Q-values, revealing the inter-chain distance d (Figure 1). Exploiting the scattering sensitivity to structural features at different length scales, makes possible to determine how dynamical and topological arrests affect correlations at segmental and network levels. This was realized by following the thermal evolution of inter-cluster and inter-chain correlations (‘microscopic dilatometry’). At the inter-chain level, chains expand obeying the same expansion coefficient throughout the entire viscoelastic region, i.e., both in the elastomeric regime and in the liquid regime (Figure 2a). The onset of liquid-like behaviour is only apparent at the mesoscale, where the scattering reveals features related to the reorganization of the network triggered by bond exchange events (Figure 2b). This is manifested by different expansion of the clusters including the dynamic bonds below and above a temperature identified as the “microscopic” topological transition temperature Tv. Proper thermal (aging-like) protocols were proposed, and revealed signatures of the ‘microscopic’ topological transition also by a ‘macroscopic’ technique such as the Differential Scanning Calorimetry (DSC) (Figure 2c), opening the door to identify this important transition temperature also by a standard technique.
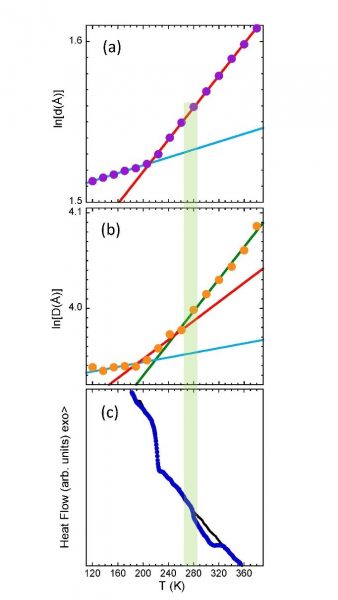
Figure 2: Temperature dependence of the natural logarithm of the average interchain distance, ln(d) (a) the average intercluster distance ln(D) (b) and heat flow (c). Calorimetric results were obtained on heating after fast cooling (black lines) and after slow cooling (blue points). Shadowed red area indicates the location of Tv.



