Power discontinuity and shift of the energy onset of a molecular de-bromination reaction induced by hot-electron tunneling
Understanding the structural and chemical stability of organics under applied electrical bias is fundamental to further progress in (electro)-catalysis and (opto)-electronics. This work addresses the dissociation mechanism of halogen atoms and reports on its sharp dependence on the applied electrical power and molecular density of states.
Regardless of the optical or thermal excitation or field-driven injection of electrons, their interaction with organic structures is critically important for the development of many applications. The immediate, albeit transitory, electron occupation of well-defined molecular orbitals, or the related energy exchange process, is beneficial for the activation of chemical reactions. However, for the same reason, the electron interaction itself constitutes one of the main limits to the structural stability of organics in (opto)-electronics.
This collaborative work between the Donostia International Physics Center (DIPC), the University of the Basque Country (EHU/UPV) and the Material Physics Center (MPC) addresses the mechanism of molecular dissociation under applied bias by injecting electrons in tunneling conditions. Specifically, the researchers have correlated the energy of debromination of an aryl group with its density of states in a self-assembled dimeric structure of 4’-bromo-4-mercaptobiphenyl adsorbed on an Au(111) surface. The authors have observed that the electron- energy range where the molecule is chemically stable can be extended, shifting the bias threshold for the rupture of the C–Br bond continuously from about 2.4 to 4.4 V by changing the electron current. Correspondingly, the power needed for the dissociation changes; however, surprisingly, it drops sharply to about 20% of the original value at 3.6 V. The abrupt change identifies two different reaction regimes and the contribution of additional molecular resonance states.
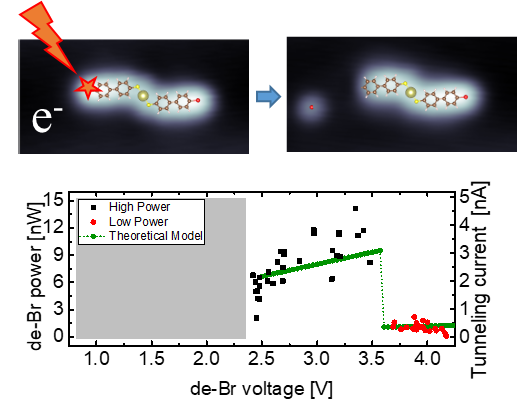
Figure: Graphical representation of the electron induced dissociation of Br atoms in the Au(Br-MBP)2 complex. The reaction onset can be controlled and shifted for almost 2eV from 2.4eV, however, the reaction power changes sharply when additional molecular resonances are involved.



