Quantum Tunneling Rotors Unravel Gas-uptake Pathways in Metal-organic Frameworks
Metal-organic frameworks can store sizable quantities of gaseous species, with immediate applications in the sequestration of greenhouse or flue-gas emissions from industrial processes. By virtue of their carbon-based nature, their inherent flexibility also leads to novel sorption mechanisms, which still defy a robust understanding at the atomic and molecular levels.
To address the above challenge, this work has capitalized from recent developments in simultaneous neutron diffraction and spectroscopy, to study in-situ and at an unprecedented level of detail the adsorption of host species in the zeolitic imidazolate framework ZIF-8. Extensive first-principles calculations were also deployed to interpret the experimental results in a quantitative manner. The nanopores in ZIF-8 are decorated with an ordered ensemble of methyl (-CH3) functional groups where the three protons become indistinguishable via quantum tunneling (Figure). This phenomenon gives rise to a well-defined energy-level structure uniquely sensitive to subtle changes to the potential energy landscape inside the pore in the absence and presence of guest species. The bottom panel in the figure shows experimental results for the case of argon. With the neutron data, we can dissect the adsorption isotherm into the filling of distinct sites. The trends at low pressures show differences relative to higher-pressure conditions, particularly in terms of the inability in the former case to access the four-membered rings within the porous structure. In both situations, other adsorption sites on either side of these tight rings are accessible and occupied when a sufficient amount of gas is supplied. Unveiling this behavior with a genuinely quantum probe is key to understanding the gas-uptake response: the steps seen in the adsorption isotherms, tunneling spectra, and diffraction patterns only occur after the two most readily accessible sites have been filled in a sequential manner. Structurally, this process requires a rotation of the organic linker within the framework, to make other sites accessible via a dynamic ´gate-opening´ mechanism. Progressively, it also leads to a ´gate-blocked´ structure where the linkers are no longer able to rotate into a ´gate-closed´ position owing to the presence of the adsorbate. These effects are accompanied by changes to the long-range order of the framework (lower-right panel of the figure). In addition to these much-needed physical insights, this study also offers a glimpse at the yet-to-be-tapped scientific potential for materials research of the European Spallation Source, soon to become operational in Lund, Sweden.
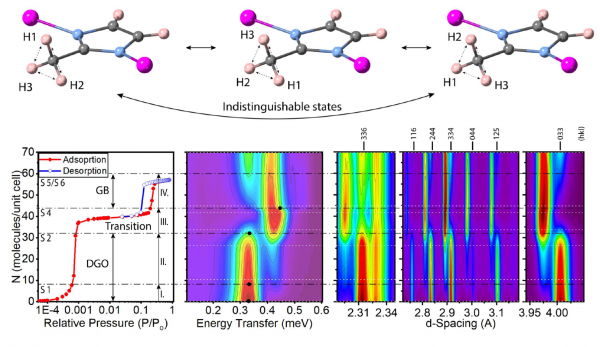
Figure: (Top panel) Schematic diagram of the methyl-group motif in ZIF-8, highlighting the three indistinguishable protons via rotation about the carbon-carbon axis. (Bottom panel) Experimental results for the case of argon – from left to right: adsorption-desorption isotherms; methyl-tunneling inelastic neutron-scattering spectra; and neutron diffraction.



