Simultaneous ignition of the CO oxidation at all facet planes of a curved platinum surface
CO oxidation (2CO + O2 → CO2) on platinum metal surfaces is a model heterogeneous gas/surface catalytic reaction. Here, researchers investigated CO oxidation at different Pt crystal planes around the (111) direction, using a curved Pt surface. They surprisingly found that the ignition occurs simultaneously at all surface facets.
Platinum (Pt) is of upmost importance as a catalyst for car exhaust cleaning or for the water gas shift reaction, whereas Pt crystal surfaces are model systems for investigating the catalytic CO oxidation at the atomic scale. During decades, researchers have studied the sequential and simultaneous interaction of CO and O2 with Pt under high vacuum conditions, providing a detailed description of fundamental steps in the reaction: O2 dissociation, CO and O chemisorption, and CO-O interaction. Lately, a deeper insight is being gained through techniques operating at millibar pressures, such as Near-Ambient Pressure X-ray Photoemission Spectroscopy (NAP-XPS). All these studies agree on the fundamental picture: the abrupt transition at “ignition”, from the low-temperature poisoning stage, when CO covers the surface and blocks the reaction, to the high-temperature active stage, when CO is displaced from the surface by chemisorbed oxygen. However, the mechanism of such CO → O transition, and the differences among crystal planes, of enormous relevance in nanoparticle catalysis, remain unknown.
Researchers from the Nanophysics Lab of CFM, and Lund investigated the ignition of the CO oxidation reaction using a curved Pt surface (Figure a). Experiments combined Planar Laser Induced Fluorescence (PLIF) of CO (Lund, Figure b), with operando NAP-XPS (Brookhaven synchrotron, Figure c). PLIF images the ignition moment in the entire curved sample, showing that it occurs in all points simultaneously, irrespective of the reaction parameters. NAP-XPS identifies chemical species across the same surface at fixed temperature, providing the clues for this surprising behavior: as surface CO concentration decreases near ignition, minor amounts of O build up at the subsurface. DFT theory indicates that a CO-Pt-O complex develops that binds CO molecules to (111) terraces strongly, equaling CO adsorption energy in flat and stepped planes, and explaining why CO desorbs at the same temperature from all facets in the curved Pt surface.
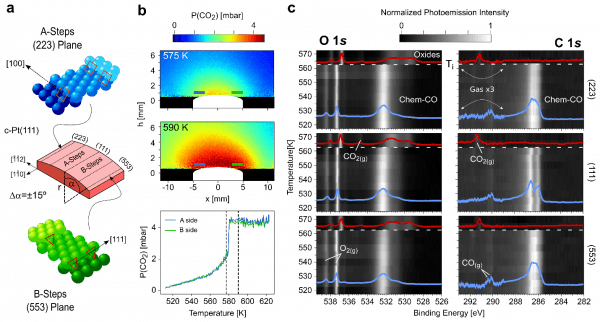
Figure: Simultaneous ignition of the CO oxidation on a curved Pt surface. (a) Sketch of the c-Pt(111) curved crystal sample featuring A-type and B-type vicinal surfaces. (b) Top-middle, two-dimensional snapshots of Planar Laser Induced Fluorescence (PLIF) of CO2 for a 6 mbar CO:O2 mixture in front of the c-Pt(111) surface, right before (575 K) and after (590 K) light-off, illustrating the simultaneous ignition of the CO oxidation at all points. Bottom, CO2 PLIF signal measured at the stepped A- and B-edges of the sample, marking the ignition at ∼580 K. (c) O 1s (left) and C 1s (right) photoemission intensity images measured at three different points on the c-Pt(111) sample exposed to ∼1 mbar CO:O2 during a heating cycle. Vanishing of CO and emergence of oxygen-related species mark an abrupt, simultaneous ignition of the reaction.



