SINGLE-CHAIN NANOPARTICLES: OPPORTUNITIES PROVIDED BY INTERNAL AND EXTERNAL CONFINEMENT
Folding individual synthetic polymer chains at high dilution by means of multiple intra-chain (reversible or irreversible) bonding interactions produces single-chain nanoparticles (SCNPs). The size of these soft nano-objects can be easily tuned in a typical range of 3–30 nm. Only a rough analogy exists between the process of SCNP formation and the precise, specific folding of a polypeptide chain to its native, functional state (e.g., enzymes). In fact, most SCNPs result in a typical morphology in solution more akin to those displayed by intrinsically disordered proteins (IDPs) than those found in compact, globular enzymes. Strategies to induce globule formation in SCNPs were also investigated, most of them based on tuning the hydrophobic/hydrophilic balance of the SCNP precursor. Even following an imperfect folding process, the conformational degrees of freedom in SCNPs are severely restricted, giving rise to notorious local domain formation and, hence, to topological self-confinement effects.
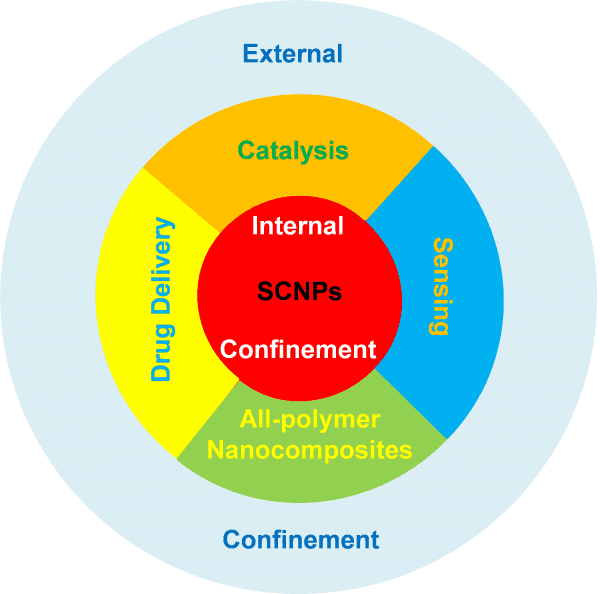
Both self-confinement and external confinement offer a plethora of opportunities to tune the properties of SCNPs for a variety of practical applications.
Both self-confinement –a class of topological effect resulting from the folding of a linear synthetic polymer chain to a SCNP– and external confinement –as exerted over SCNPs by imposing geometrical constraints as well as by other macromolecules or SCNPs in a concentrated solution, on a highly decorated surface or in the bulk (melt) state– offer a plethora of opportunities to tune the properties of SCNPs for a variety of practical applications. Some of these potential uses, especially those related to self-confinement in SCNPs, have already been established. In fact, some SCNPs do mimic the outstanding properties of antimicrobial polypeptides and both the size and function of certain metalloenzymes, and intrinsically disordered and structural proteins. Other uses will presumably be developed by exploiting external confinement of SCNPs as an extra parameter for tuning the properties and functionality of SCNPs: from innovative purification techniques and improved all-polymer nanocomposites, to smart responsive surfaces and new topological nanostructures, among other ones.
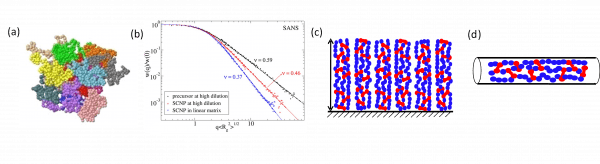
Illustration of external confinement imposed on SCNPs: (a) Crumpled globule morphology of sparse SCNPs (drawn in different colours) in a concentrated solution as revealed by MD simulations. (b) Change in conformation of SCNPs on passing from dilute solution (sparse open conformation, scaling exponent ν = 0.46) to all-polymer nanocomposites (crumpled globule conformation, scaling exponent ν = 0.37) as determined by SANS experiments. (c) Schematic illustration of arrays of SCNPs on surfaces. (d) Change in conformation of SCNPs in nanopores.



