Structure matters: asymmetric CO oxidation at Rhodium steps with different atomic packing
The CO oxidation reaction was monitored operando on a curved rhodium crystal, using Ambient Pressure Photoemission. Maps of surface species obtained within a wide range of temperatures demonstrate a step-packing-dependent asymmetry of the reaction process, proving the persistent presence of very active chemisorbed oxygen at triangular B-type steps.
The carbon monoxide (CO) oxidation (CO + ½ O2 → CO2) is an industrially relevant catalytic reaction, and accordingly, it is among the most studied processes in fundamental surface science. This has led to a wealth of research studies in vacuum, but the doubts remain on whether this reaction proceeds in the same way when changing to realistic conditions. With the advent of powerful analytical techniques that operate at high gas pressures, such as Ambient Pressure X-ray Photoemission (AP-XPS), the “pressure gap” has been reduced significantly. However, a “materials gap” persists, since most AP-XPS studies are carried out using single crystal surfaces, which contrast with the multi-facet structure of technologically relevant nanoparticles.
Curved crystals are a simple approach to bridge the materials gap between single crystal surfaces and nanoparticle catalysts, as depicted in Figure 1. This shows a Rh crystal “curved” around the (111) direction, leading to a smooth variation of the crystal plane at each point. Such geometry allows the different crystal facets to be sequentially probed by scanning the X-ray beam in AP-XPS. With this approach, we have investigated the effect of A-type (square geometry, left side of the crystal) and B-type (triangular geometry, right side of the crystal) atomic packing of steps on the catalytic CO oxidation on Rh at millibar pressures.
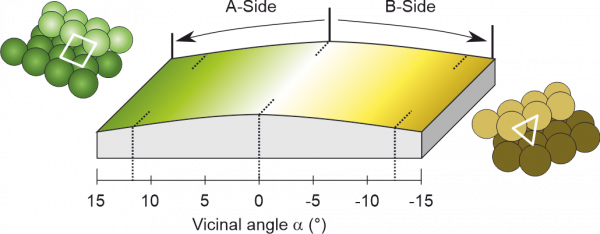
Figure 1: Schematic description of the curved Rh(111) sample used to probe the catalytic activity of Rh atomic steps with square (A-type) and triangular (B-type) symmetry in a simultaneous way, via Near Ambient Pressure Photoemission.
In this work, researchers from CFM and other international institutions have exposed the Rh curved sample of Figure 1 to a reactive CO+O2 atmosphere, and have identified the sequence and thermal evolution of surface chemical species across the different crystal planes around the reaction light-off. A striking asymmetry is observed between A- and B-type steps both during the ignition and at the fully active reaction stages. Figure 2 shows XPS maps of chemical species at different temperatures as a function of the local crystal plane. The maps acquired during the ignition prove the partial CO-depletion and O-accumulation at B-steps, triggering the earlier B-step ignition. In the active stage of the reaction, the low-active Rh oxide builds up at A-steps (square atomic packing), while B-steps (triangular atomic packing) retain the active chemisorbed oxygen species during reaction conditions.
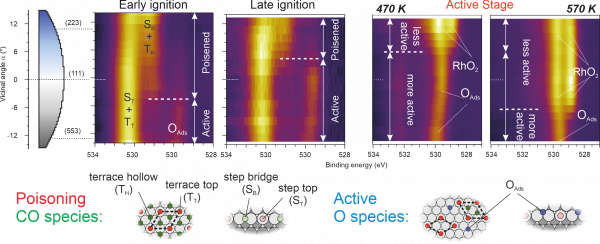
Figure 2: Map of surface chemical species across different crystal planes (vicinal angle α on the curved Rh(111) sample), measured at near-ambient pressure (0.5:0.5 mbar of CO:O2), at different temperatures around the ignition point and the active stage. A marked A-B asymmetry is observed at all reaction stages.



