Thioetherification of Br-mercaptobiphenyl molecules on Au(111)
Scanning tunneling microscopy has emerged as a powerful tool that significantly contributes to our understanding of chemical reactions, particularly in visualizing catalytic reaction steps on surface templates. These investigations have played a crucial role in clarifying the reaction steps leading to the synthesis of nanostructures with desired shapes and electronic properties, which are essential for advancing quantum technologies.
Despite its relevance and success in unravelling reaction steps, most investigations focused on synthesizing polymeric nanostructures using molecular precursors with a single functionalized reacting group. For example, graphene nanostructures are primarily formed on metal surfaces through the thermal activation of molecular de-halogenation followed by carbon-carbon coupling between adjacent molecules. Thiols, instead, most often dehydrogenate leading to strong chemical bonds to metal surfaces. The strength of this bond is such that thermal activation rather induces the detachment of chalcogen atoms from organic structures. Investigations exploring on-surface reaction steps between molecular precursors holding simultaneously these two groups have not been undertaken yet.
In a collaborative effort between CFM, Ikerbasque, and DIPC, molecular C-S bonds were successfully formed on Au (111) surfaces using molecular precursors functionalised with both halogen and a sulfhydryl group. This introduces an important novelty to the field of surface synthesis. The reaction leads exclusively to the thioetherification of the molecules despite the two mentioned competing reactions. Extended polymeric chains, characterized by new chemical C-S bonds are observed upon thermal activation of 4′-Bromo-4-mercaptobiphenyl molecular precursors adsorbed on the Au surface.
The authors identified four reaction steps involving sulfhydryl or bromine molecular groups leading to intermolecular C-S bond formation, through scanning tunneling microscopy, spectroscopy, and first-principles calculations. The study shows that to form the thioether polymer and overcome the common competitive formation of C-C bonds, two of these reaction steps, namely the dehalogenation and dissociation of the S-Au bond, must occur simultaneously. This research provides insights into such polymeric structure synthesis, revealing changes in precursor electronic properties upon bonding and molecular precursor length.
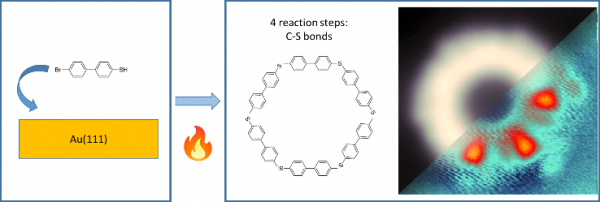
Figure: Schematic representation of the thermally induced thioetherification process of 4′-Bromo-4-mercaptobiphenyl (Br-MBP) molecules on the Au (111) surface. The presence of S atoms strongly modulates the electronic properties of the phenyl chain.



