Spectroscopy at atomic scale group
Chemical reactions at nanoscale
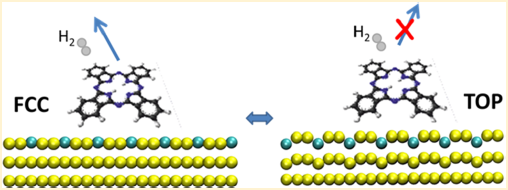

Observing chemical reactions at nanoscale is a fundamental issue of nanoscience. The detailed knowledge of the mechanisms and reactions steps leading to its chemical reaction either induced by catalytic process or induced by failure of the molecular structure is of paramount importance for solid-state nanochemistry. In our work, for example, we have tried answering the questions: "to which limits is a molecular structure stable under electron transport?" and "what is the mechanism leading to its failure? Indeed, despite most characterized reactions on surfaces are thermally activated, the dissociation reactivity of molecules can be achieved also by electrochemistry. Energy transferred to specific molecular bonds during electron transport can results in bond breaking. Our recent work article, shows that the molecular stability in a voltage region of interest of applications can be tuned and considerably extent before a debromination reaction occurs. The power associated to the reaction however become considerbly smaller and drops to only 20% (!!) of its original value, when a second molecular resonance comes into play.
However, a reaction can be catalized by the contact with a surface. Recently, we have been able to shed some light on the mechanistic steps of a debromination reaction, which precede the Ullmann cross-coupling reaction and the formation of new covalent C-C bonds. Currently, the Ullmann cross-coupling reaction is undergoing a considerable revival. Its application allowing the reliable synthesis of extended polymers has opened a fruitful path in surface chemistry, leading to the formation of polyphenyls, graphene structures of well-defined shape, or covalently assembled structures with engineered electronic and functional properties
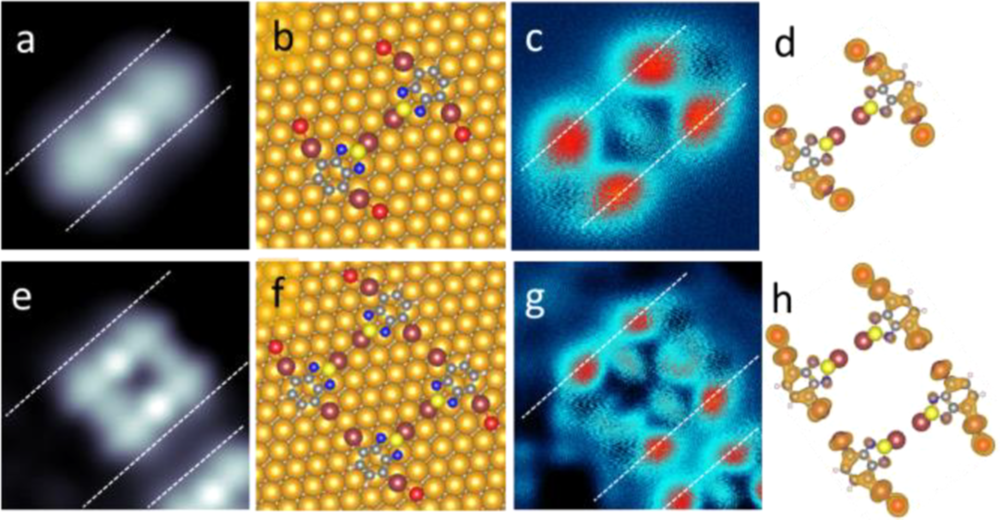
Indeed, while there is general agreement that at a certain point of the reaction of Cu-catalyzed dehalogenation reaction, a copper-coordinated structure is formed, the mechanism responsible for the initial debromination step was still under debate. In our work, we have characterized a prototypical molecule, namely 4,7-dibromobenzo[c]- 1,2,5-thiadiazole (2Br-BTD), deposited on a Cu(110) surface. We have demonstrated the oxidative addition of Cu atoms leading to a −C−Cu−Br metal− organic complex. The scission of the strongly bound bromine atoms requires the cooperative action of neighboring complexes resulting in the formation of Cu coordinated BTD structures article .
If it is true that a chemical reaction can be induced by a metal contact, it is also certain that not all the "contact sites" with a surface are equally efficient to promote selectivity and reaction specificity. The reaction steps often relate to the elements and the reaction sites. However, not always this information is available. Surface nanochemistry allows the visualization and characterization of intermediate reactions steps, an unprecedented step forwards in the understanding of the catalytic reaction processes.
The detailed knowledge of the relation between the atomic structure and chemical reactivity is of paramount importance for solid-state nanochemistry. The efficiency of chemical reactions on surfaces is traditionally related to their lattice structures. Nanostructures of well-defined shape and size as well as surface step border have been shown as privileged reaction sites. We have demonstrated that lattice distortions of supported two-dimensional layers leading to periodic out-of-plane displacement of single atoms offer an alternative strategy to surface functionalization, scarcely investigated so far. Specifically, we show that the variable buckling geometry of a GdAu2 Moiré overlayer supported on the Au(111) surface exposes specific single-atom sites (article) that control the dehydrogenation process of phthalocyanine (H2- PC) molecules, limiting their reactivity to about 1/3 of the monolayer. This surface reactivity is thus determined by lattice mismatch between the substrate and the alloy layer which lead to an outward displacement of distinct Gd sites. These atomic sites promote the selective dehydrogenation of H2-PC molecules article .
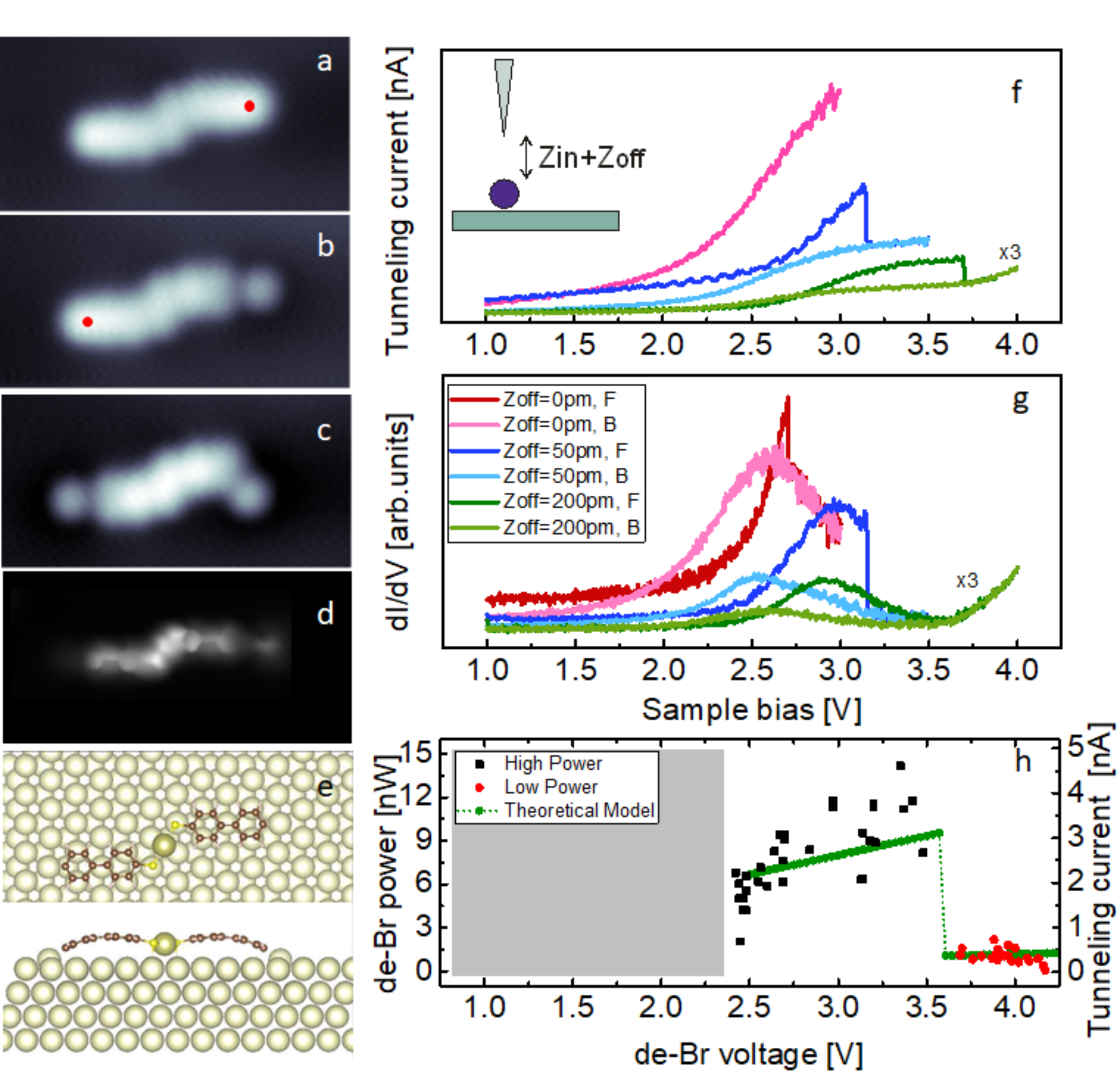
Fingeprints of a debromination event: topographic and spectroscopic evidences. article
Spectroscopic fingerprint of Br atoms in self-assembled structures showing that the fingerprint of the bromine atoms in the density of states (red dots) is uniquely observed at the outermost side of the molecular ensemble. article
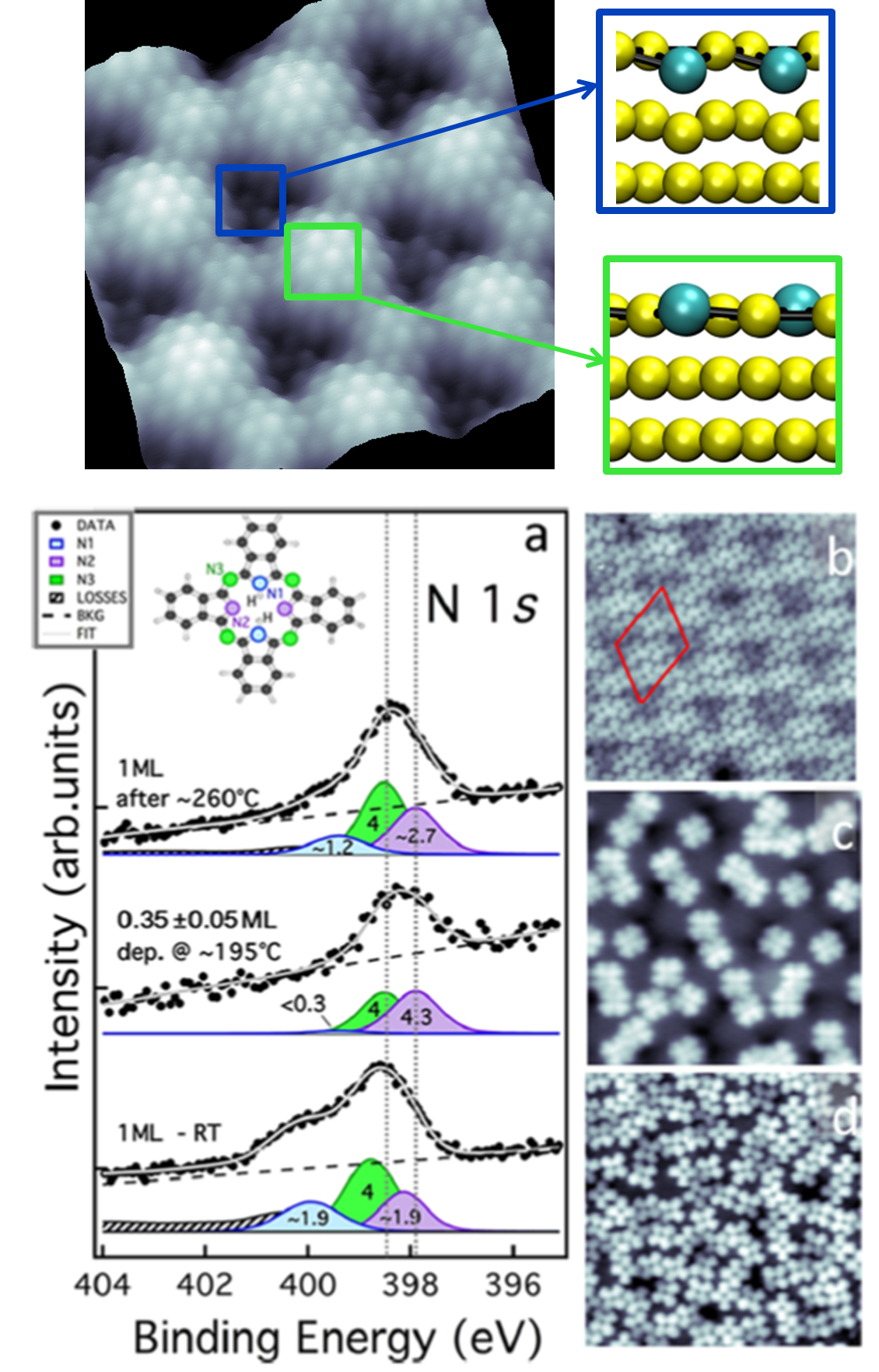
Topographic image of GdAu2/Au(111) and model of the periodic displacement of the Gd atoms (green). Selective dehydrogenation of H2-PC molecules on the GdAu2 layer. article
Ana Barragan, Roberto Robles, Nicolas Lorente, Lucia Vitali
Nanoscale 13, 15215 (2021)
article
Matteo Farnesi Camellone, Alexander Correa, Ana Barragan, Maddalena Pedio, Stefano Fabris, Cinzia Cepek, Lucia Vitali
J.Phys.ChemC.123.6496 (2019)
article
Ane Sarasola, Ana Barragan, Lucia Vitali
J.Am.Chem.Soc 2018, 140, 15631
article
Alexander Correa, Matteo Farnesi Camellone, Ana Barragan, Abhishek Kumar, Cinzia Cepek, Maddalena Pedio, Stefano Fabris and Lucia Vitali
Nanoscale 2017,9, 17342-17348
article
A.Basagni, G.Vasseur, C.A. Pignedoli, M.Vilas-Varela, D.Peña, L. Nicolas, L.Vitali, J.Lobo-Checa, D. G. de Oteyza, F. Sedona, M. Casarin, J. E.Ortega, M.Sambi
ACS nano 10, 2644 (2016)
article
R. Ohmann, L. Vitali, K. Kern.
Nano Letters 10, 2995-3000 (2010)
article
L. Vitali, G. Levita, R. Ohmann, A. Comisso, A. De Vita, K. Kern
Nature Materials 9, 320-323 (2010)
article
L.Vitali, M.G.Ramsey, F.P.Netzer
Physical Review B 57 , 15376-15384 (1998)
article