Green Chemistry Catalysts
|
For a
better connection to industrial or technology
applications, Surface Science is required to
overcome the “pressure” and the "material" gap, that
is, to adapt to ambient or liquid conditions, at
which one can properly simulate industrial processes
and devices “in-operando”. In the last few years the
most powerful Surface Science techniques have
evolved towards this direction. The Surface
Electrochemistry group of the Nanophysics
Lab has initiated a research line to explore
basic problems of electrocatalysis and on crystal
surfaces, combining standard ultra-high-vacuum
characterization, Near-Ambient Pressure
Photoemission (NAP-XPS) experiments, and Electrochemical
cell. |
|
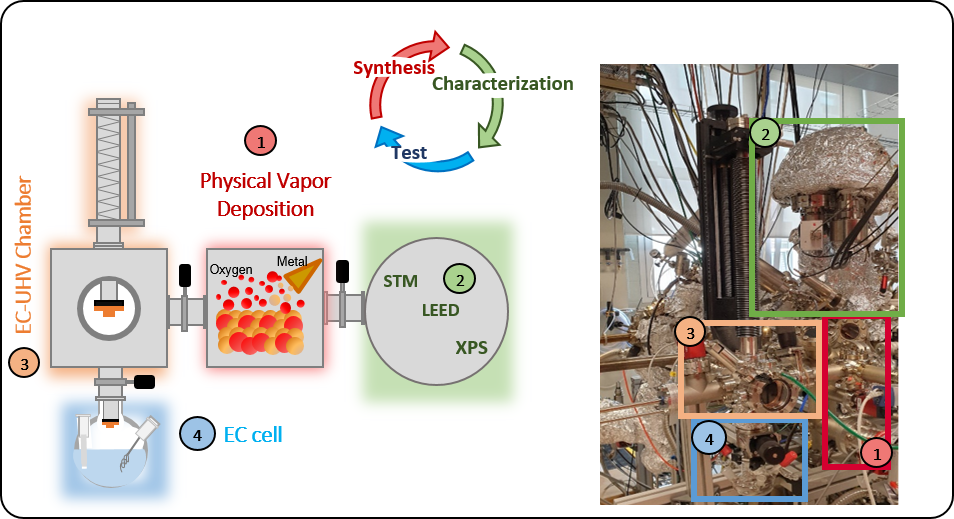
Our customized
experimental set-up enables structural,
chemical and electrochemical characterization on
exactly the same sample, by allowing the
transfer of the catalyst from ultra-high vacuum
(UHV) –compatible with surface science techniques-
to an electrochemical cell in a controlled argon gas
atmosphere. This optimized approach enables the
direct correlation between the surface composition
(X-Ray photoemission spectroscopy, XPS) and
structure (Low energy electron diffraction, LEED,
and Scanning tunneling microscopy, STM) at the
atomic scale, and the macroscopic response of the
catalyst (Cyclic Voltammetry, CV, Linear Sweep
Voltammetry, LSV, and Chronoamperometry, CA.). Main responsibles: ENRIQUE ORTEGA & SARA BARJA & FREDERIK SCHILLER |